A Re/Insurance View Of GLP-1s
(Glucagon-Like Peptide 1 Receptor Agonists)
GLP-1 is a natural hormone produced by your body after eating, to help regulate your blood sugar and lower your appetite. New GLP-1 drugs supplement your natural production, to treat Type 2 diabetes and lower risks of related complications such as heart disease and kidney damage.
Research has discovered GLP-1 drugs are also effective weight-loss tools, and prescriptions are rising fast. By 2024 one in eight U.S. adults had already taken GLP-1 at some point and the global market is projected to triple in the next five years, driven by high and rising levels of type-2 diabetes and obesity, aging populations, urbanization and public health initiatives:
Global GLP-1 Receptor Agonist Marker
(2018-2030E)
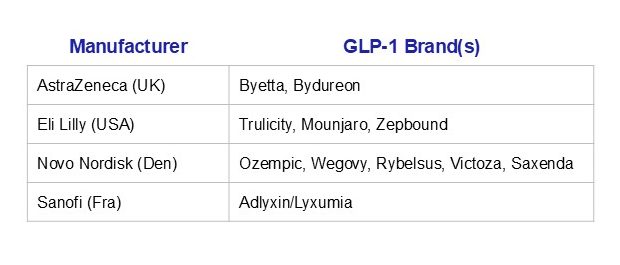
Global pharmaceutical companies dominate today’s production:
With ~300 clinical trials around the world, research continues into applications to other metabolic disorders, cardiovascular and kidney disease and neurological conditions. Innovations are expected to focus on safety, efficacy, affordability and convenience (oral applications, auto injectors etc).
Regulatory approval follows rigorous trials, and some GLP-1 drugs are approved for obesity management, others for type 2 diabetes. In 2024 Wegovy was the first drug approved by the FDA to treat cardiovascular disease in people with obesity.
The long-term effects of GLP-1 are not yet fully clear, but documented side effects observed within a year of usage include (in descending order of frequency) digestive system issues, abdominal and pelvic pain, nausea and vomiting, hypertension and dehydration.
Re/insurance Implications
However, good news for weight-watchers and type-2 diabetics may concern re/insurers. If significant side effects materialize, the high (and rising) sales of GLP-1 for weight loss treatment will likely attract the attention of plaintiff lawyers and litigation funders. We know (from similar, prior claims) that the date of loss can vary, and we must carefully structure coverage to avoid opening multiple years to the unintended impact of the same/similar ingredient.
Which brings us to Ozempic’s Multi District Litigation. Novo Nordisk, Ozempic’s manufacturer, faces over 2,000 people claiming they developed severe gastrointestinal conditions after taking the drug. The Master Complaint claims failure to provide sufficient warnings about the increased risk, breach of warranties, fraudulent concealment, unfair trade practices, strict product liability, misrepresentation, negligent design and on and on and on….
We don’t know the ultimate number of claims, but medical treatment for diabetes and obesity management are evolving rapidly, with GLP-1 drugs at the forefront.
We don’t know that distributors will be brought into litigation, but the Opioid playbook suggests they might.
We know that patented drugs put their manufacturers at the front of any litigation, while generic manufacturers in the U.S. have some limited protection around labeling (2011’s Mensing rule).
In Summary
Prescriptions are up, innovation is rapid, and litigation Is underway. We have seen this movie (OxyContin/Purdue/Sackler). Given the scale of potential exposure, we will continue to weigh the implications of GLP-1 drugs cautiously.
Please contact Keith Trigg, Global Portfolio Casualty Leader or Maryam Haji, PhD, Global Head of Research with any questions or comments on this topic.
DISCLAIMER: Reproduction in any form without permission of TransRe is prohibited. The material and conclusions contained in this document are for information purposes only and TransRe offers no guarantee for the completeness of its contents. Statements in this document may provide current expectations of future events based on certain assumptions. These statements involve known and unknown risks, uncertainties and other factors which are not exhaustive. Although TransRe makes reasonable efforts to obtain information from reliable sources, TransRe does not guarantee the accuracy or completeness of the information given or any forward-looking statements made. TransRe undertakes no obligations to revise or update any statements, whether as a result of new information, future events or otherwise, and in no event shall TransRe or any of its affiliates or employees be liable for any damage or loss arising in connection with the use of the information relating to this document.
Legal
Reproduction in any form without permission of TransRe is prohibited.
The material and conclusions contained in this document are for information purposes only and TransRe offers no guarantee for the completeness of its contents.
Statements in this document may provide current expectations of future events based on certain assumptions. These statements involve known and unknown risks, uncertainties and other factors which are not exhaustive. Although TransRe makes reasonable efforts to obtain information from reliable sources, TransRe does not guarantee the accuracy or completeness of the information given or any forward-looking statements made.
TransRe undertakes no obligations to revise or update any statements, whether as a result of new information, future events or otherwise, and in no event shall TransRe or any of its affiliates or employees be liable for any damage or loss arising in connection with the use of the information relating to this document.
The Best’s Company Report(s) reproduced on this site appear under license from A.M. Best and do not constitute, either expressly or implied, an endorsement of (Licensee)’s products or services. A.M. Best is not responsible for transcription errors made in presenting Best’s Company Reports. Best’s Company Reports are copyright © A.M. Best Company and may not be reproduced or distributed without the express written permission of A.M. Best Company. Visitors to this web site are authorized to print a single copy of the Best’s Company Report(s) displayed here for their own personal use. Any other printing, copying or distribution is strictly prohibited.
Best’s Ratings are under continuous review and subject to change and/or affirmation. To confirm the current rating, please visit the AM Best web site, www.ambest.com.